About Mercy Research
Mercy has a long history of engaging in clinical trials research and outcome-based research. These roots have helped to develop our health care services for the communities we serve. On July 1, 2016, Mercy pioneered a new model for community health-based research when Mercy Research was formed, consolidating all research activities across Mercy into a stand-alone, not-for-profit entity.
Mercy Mission: As the Sisters of Mercy before us, we bring to life the healing ministry of Jesus through our compassionate care and exceptional service. Mercy Research fulfills this mission by offering innovative research strategies in the communities we serve.
Our Vision: Mercy Research improves health care for our patients and communities by offering clinical research studies and exploring solutions to health care challenges.
Our Purpose: Mercy Research is committed to advancing the quality of health care for individuals and communities by being a leader in innovative clinical, scientific and health services research. We will achieve this goal through participation in clinic trials and development of new medical products, medical treatments and delivery systems that transform lives and improve overall community health, in keeping with the mission of mercy to bring the healing ministry of Jesus to life, through compassionate care and exceptional service.
Consider making a donation to help Mercy Research continue the innovative work of clinical research at Mercy.
Mercy Research Areas of Service
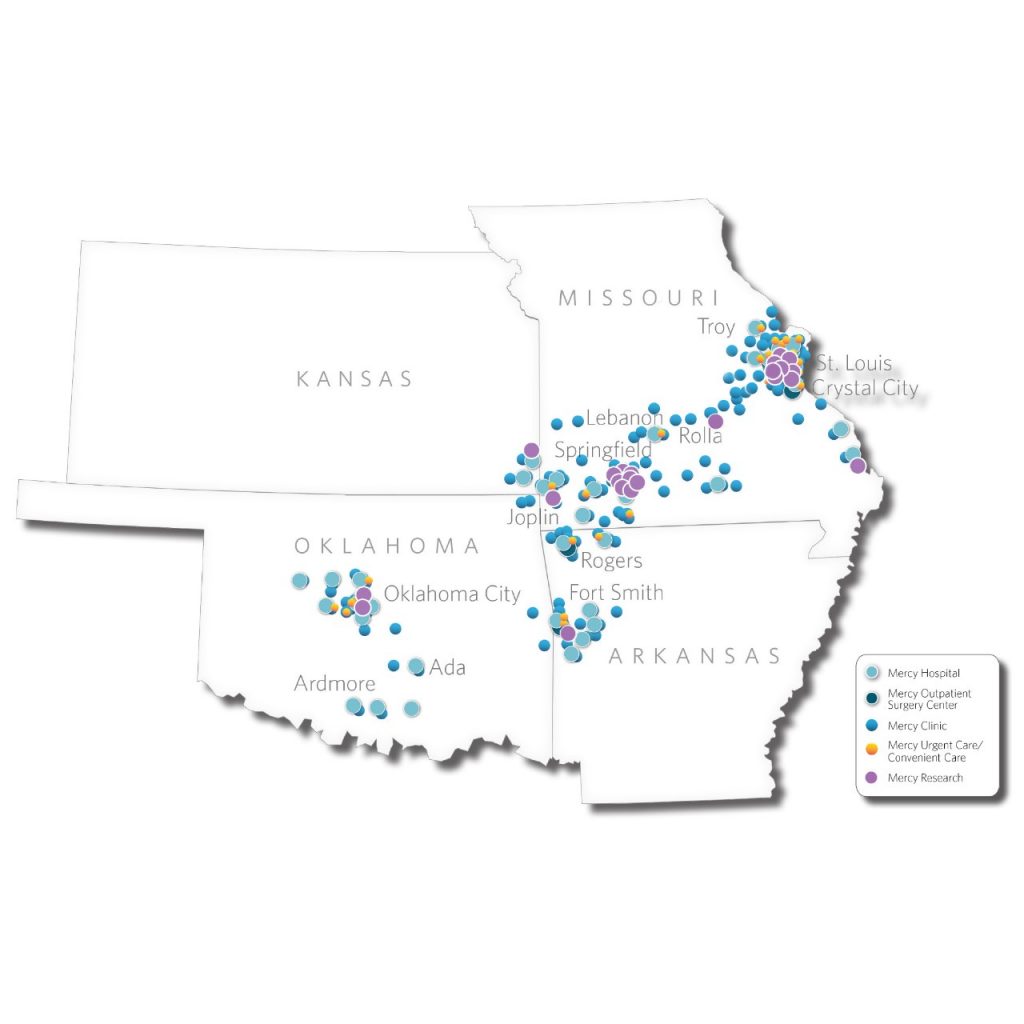
Mercy Research is one of the nation’s largest fully integrated, community health system-based research organizations. We are part of Mercy – named one of the top five large U.S. health systems for four consecutive years by IBM Watson Health. Mercy has an extensive regional presence over four states:

- 2x
- 1.75x
- 1.5x
- 1.25x
- 1x, selected
- 0.75x
- 0.5x
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Mercy Research: Improving Patient Health
JoAnne Levy - Vice President
Learn how Mercy Research brings trial opportunities and hope to more people, in more places.
The Resources of Mercy
Mercy offers a millions-strong, diverse patient population with single touch-point coordination. Mercy is one of the nation’s most highly integrated, multi-state health care systems, including more than 40 acute care, managed and specialty (heart, children’s, orthopedic and rehab) hospitals, convenient urgent care locations, imaging centers and pharmacies. Mercy has 900 physician practices and outpatient facilities, more than 4,000 Mercy Clinic physicians and advanced practitioners and 40,000-plus co-workers serving patients and families across four states. In addition, Mercy Virtual commercially serve providers and patients from coast to coast.
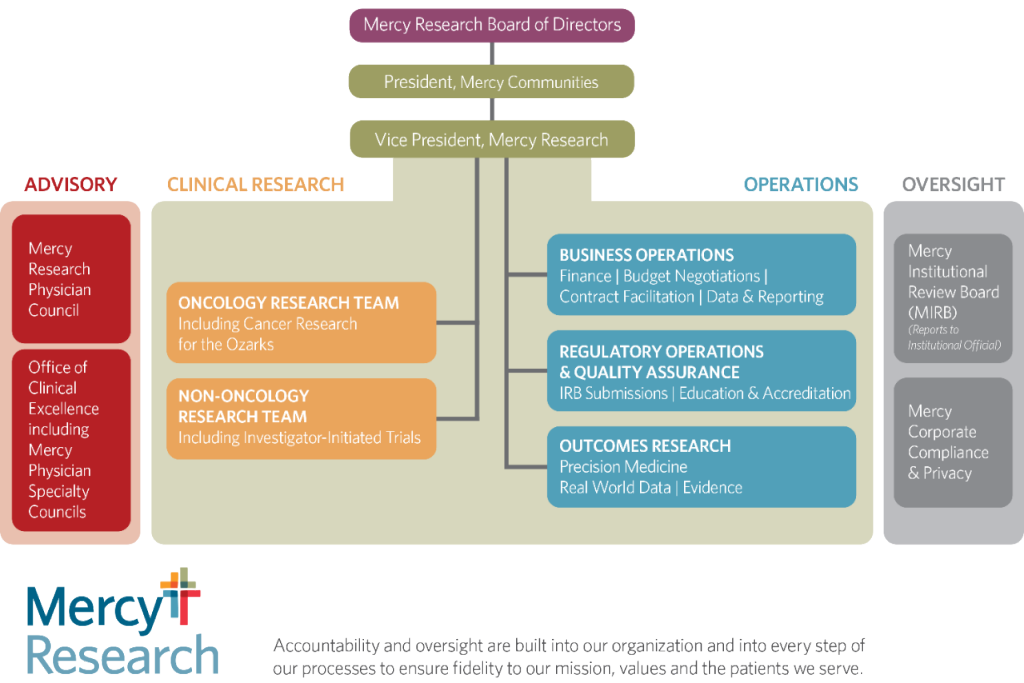
Organizational Structure
In 2016 Mercy centralized all research activities across our service areas into a stand-alone, not-for-profit entity known as Mercy Research. This type of infrastructure is unusual, especially for community health systems. Consolidating clinical research support across the entire health system allows us to standardize clinical, business and regulatory processes and use shared tools across a broad geography.
This leads to increased compliance and improved risk management. It also increases stewardship by consolidating financials and allowing for better informed and more strategic decision-making.
Mercy Research was named a Cutting Edge Award winner by the St. Louis Business Journal in 2019 for our innovative centralization of all research activities in a community health system that allows for standardization of clinical, business and regulatory processes and use of shared tools across a broad geography.

Casey Carrington
Research Marketing Manager

Casey Carrington
Research Marketing Manager
Mercy Research Physician Council
Mercy Research Board of Directors
Damon Broyles, MD Vice-Chair of the Board - Vice President, Mercy Clinical Innovations
Jay Carlson, DO Chair of the Board - Mercy Clinical Director, Cancer Care Performance Acceleration
Kimberly Creach, MD - Mercy Medical Director of Mission - Springfield
Cherian Karunapuzha, MD - Mercy Medical Director, Meinders Center for Movement Disorders - Oklahoma City
Carla Kurkjian, MD - Mercy Oncology – Oklahoma City
Gregory Ledger, MD - Mercy Endocrinology – Springfield
JoAnne Levy, JD, MBA - Vice President, Mercy Research
Jill McCart - Vice President, Accounting, Mercy Finance
Jacqueline Richmond, JD - Vice President, Mercy Deputy General Counsel
Farid Sadaka, MD - Mercy Critical Care – St. Louis
William Sistrunk, MD - Mercy Internal Medicine, Infectious Disease - Springfield
Staff Support to Mercy Research Board of Directors
Miranda Lewis – Vice President, Mercy Donor Relations and Development
Chad Smith, MD – Research Integrity Officer, Mercy Chief Medical Officer – Oklahoma Region
Courtney Wright – Mercy Director, Research Compliance
Katie Wong, JD – Executive Director - Senior Counsel, Mercy Legal
Learn More
Affiliations are enabling and advancing our research operations.
Learn MoreFor more information, contact Mercy Research.
Get ConnectedHelping Mercy providers develop and conduct their own research studies at Mercy.
Learn MoreOur clinical studies offer our patients earlier access to potentially beneficial new treatments.
Learn MoreOur clinical research brings leading-edge cancer treatments to our patients.
Learn MoreUsing real-world data and evidence to support innovation and safety.
Learn More